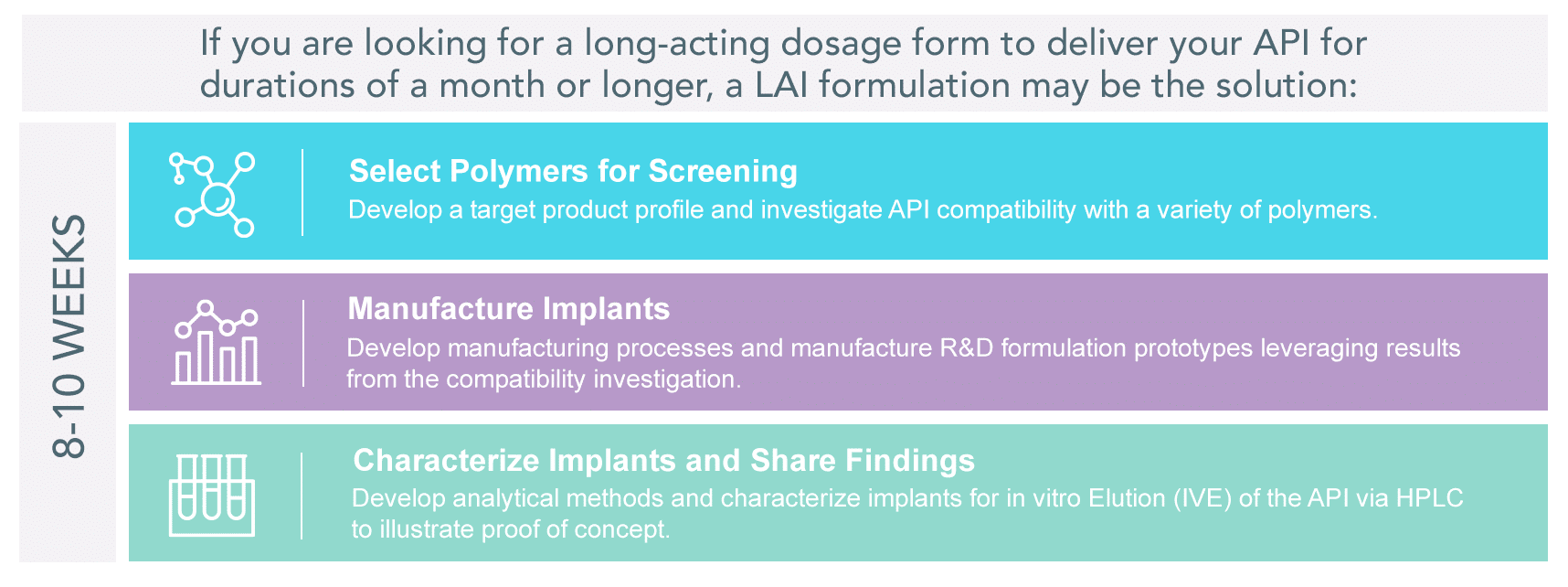
Long-Acting Implant Feasibility Program
Long-acting implantables (LAIs) are a technology that enables parenteral delivery of active pharmaceutical ingredients (APIs) for an extended duration. LAIs are typically polymer-based systems that can be used for the delivery of both small and large molecules.
Polymeric LAIs are an area of growing interest in the pharmaceutical industry as they offer benefits over other dosage forms in designing platforms for drug delivery. The marketed intravitreal LAI, Ozurdex®, was the first drug therapy approved by the FDA for long-acting treatment of macular edema, with annual sales of $400 M in 2019.

Why partner with Particle Sciences?
Particle Sciences is a leading solutions provider for long-acting implantables, and we are uniquely equipped to address the complex conceptualization, development, and manufacturing challenges that accompany the design and characterization of drug-eluting devices. To better support our customers’ LAI development, we have installed an implant extrusion line in partnership with ThermoFisher to reduce the capital expense burden of procuring equipment during early-stage development.
Our seasoned staff has experience handling highly potent APIs (hormones, steroids, cytotoxics), controlled substances (Schedule I-V), and large molecules (proteins, peptides, nucleotides). We also understand the importance of polymer control and supply when designing LAIs. To this end, Particle Sciences has established a partnership with most major suppliers of bioabsorbable polymers and we offer our extensive portfolio of Pathway™ thermoplastics polyurethanes to support biodurable LAI development.
Particle Sciences is a flexible, full-service partner capable of taking your project from early-stage development through clinical and commercial GMP production.
Frequently Asked Questions
What is a long-acting implantable?
A long-acting implantable is a parenteral product made of polymer, functional excipients, and pharmaceutical active drugs. It is typically administered using a syringe or applicator. These products consist of either bioresorbable, drug-loaded systems that erode over months to years or biodurable, non-eroding drug-loaded systems.
Why develop a long-acting implantable?
Long-acting implantable systems offer the potential for targeted drug release, lower dosage requirements, and improved patient compliance by replacing daily pills or injections. Well-designed LAIs can be tailored to a specific API and application, leading to improved bioavailability, stability, and pharmacokinetics as well as intellectual property and life-cycle management opportunities.
How are long-acting implantables supplied?
Long-acting implantables are supplied as biodegradable or biodurable systems, each exhibiting specific advantages over the other forms.
What are the differentiating factors between biodegradable and biodurable LAIs?
Biodegradable LAIs completely erode and are reabsorbed and excreted over the lifetime use of the LAI hence eliminating the need for surgical removal after completion of its intended therapeutic treatment. Biodurable LAIs remain intact throughout its lifetime of use and hence require removal after completion of its intended therapeutic treatment. This affords the benefit of facile removal in instances of adverse reaction to the pharmaceutical drug or end of treatment (birth control).
What kinds of APIs are amenable to LAI technology? Can you load large molecules into LAI?
Both small and large molecules—including proteins, peptides, and nucleic acids—have been developed into LAI systems. Highly potent compounds such as cytotoxic APIs, and DEA controlled substances are also well-suited for LAI systems.
Can you manufacture LAIs aseptically?
LAIs are typically terminally sterilized via gamma or E-beam irradiation. However, in cases where an API is not amenable to terminal sterilization (such as a biologic), Particle Sciences can evaluate the intended target product and its respective manufacturing process to assess its amenability to aseptic manufacturing.
Can you modify the polymer in LAI?
Yes—polymer selection for LAI systems is highly customizable. There are several commercially available biodegradable and biodurable chemistries that offer control over LAI properties and drug release. If an off-the-shelf polymer does not meet your target product profile, Particle Sciences can leverage our expertise in polyurethane chemistries, for developing biodurable LAIs, or our strong relationships with various suppliers of biodegradable polymers, for developing biodegradable LAIs, to develop a custom solution.
